Painful Lumbosacral Radiculopathy (LSR)
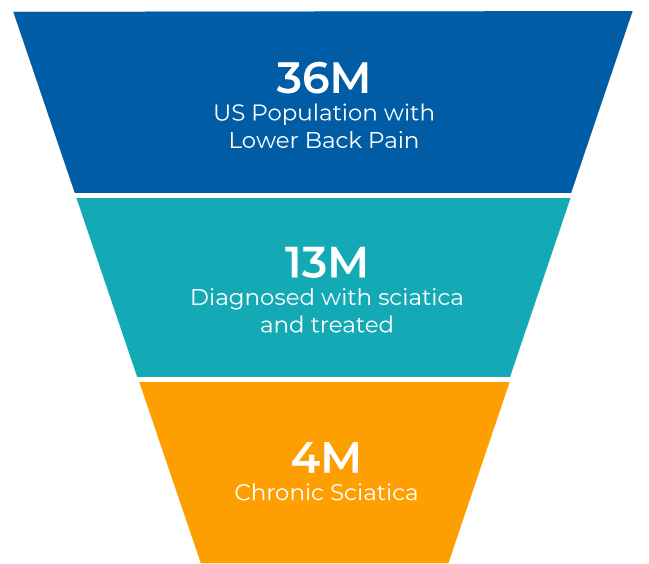
More commonly known as sciatica, chronic painful LSR is a condition caused by damage or irritation of the nerve root in the lower spine. The pain typically radiates from the lower back down the leg and can be accompanied by numbness, tingling, or weakness. For many patients, these symptoms are disabling, limiting mobility and quality of life.
Painful LSR or sciatica can be caused by many factors, the most common being a bulging or herniated disc. The condition can resolve with rest and conservative care, including physical therapy and common pain medications, but for many patients such an approach is inadequate.
LSR becomes a chronic condition when nerve inflammation and pain continue for more than three months following the onset of symptoms.